
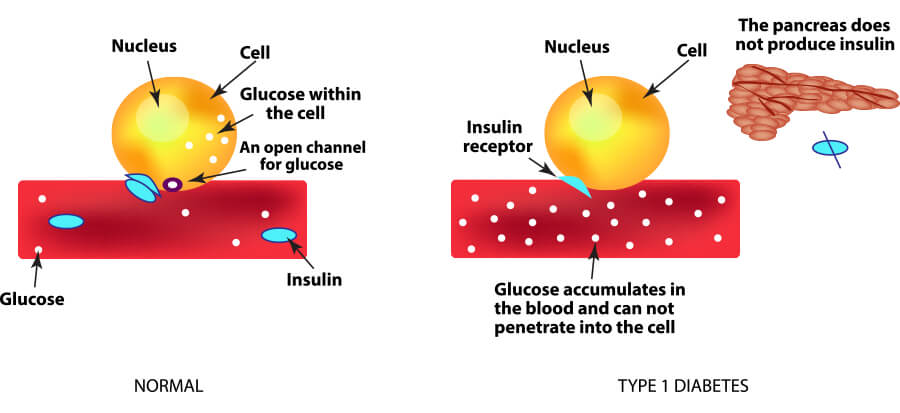
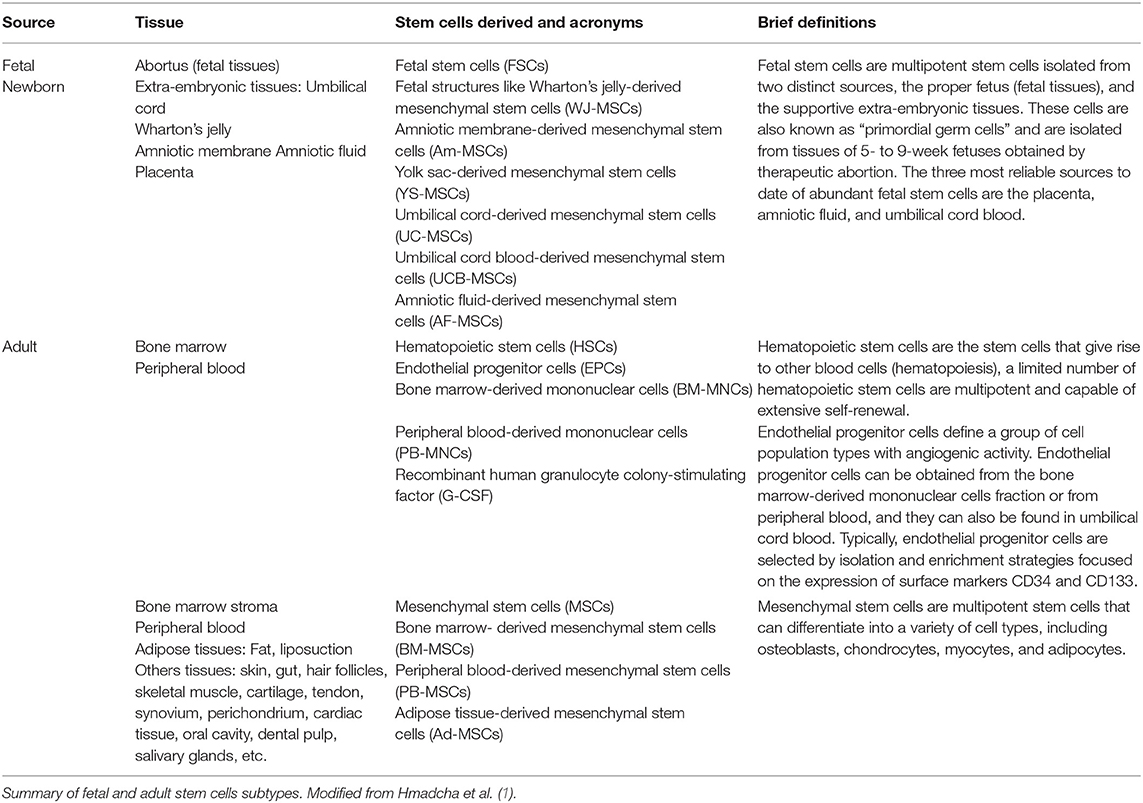
Stem cell replacement therapies overcome the barrier of finite availability, but they still face immune rejection. Transplantation of allogeneic hESC-derived insulin-producing organoids has recently entered Phase I and Phase II clinical trials. Instead, to address limitations related to supply, human embryonic stem cell (hESC)-derived β cells are being explored as surrogates for cadaveric islets. Transplantation of MSCs is found to be effective for T2D patients, but its efficacy in T1D is controversial, as the ability of MSCs to differentiate into functional β cells in vitro is poor, and transdifferentiation in vivo does not seem to occur. To improve C-peptide levels in patients with both T1 and T2 Diabetes, numerous clinical trials have explored the efficacy of mesenchymal stem cells (MSCs), both as supporting cells to protect existing β cells, and as source for newly generated β cells. For these reasons, implementation of islet transplantation has been restricted almost exclusively to patients with brittle T1D who cannot avoid hypoglycemic events despite optimized insulin therapy. Insulin independence is typically achieved upon islet transplantation, but on average just 25% of patients do not require exogenous insulin injections five years after. Despite improvements in the immunosuppressive regimen, the number of required islets remains high, with two or more donors per patient often needed. Despite its positive outcome, the impact of islet transplantation has been limited due to a number of confounding issues, including the limited availability of cadaveric islets, the typically lifelong dependence of immunosuppressive drugs, and the lack of coverage of transplant costs by health insurance companies in some countries. Since its introduction more than twenty years ago, intraportal allogeneic cadaveric islet transplantation has been shown to be a promising therapy for patients with Type I Diabetes (T1D). Diabetes Center, University of California San Francisco, San Francisco, CA, United States.


 0 kommentar(er)
0 kommentar(er)
